Clinical Trials Network
We’ve made it easier for you to find clinical trials in Canada!
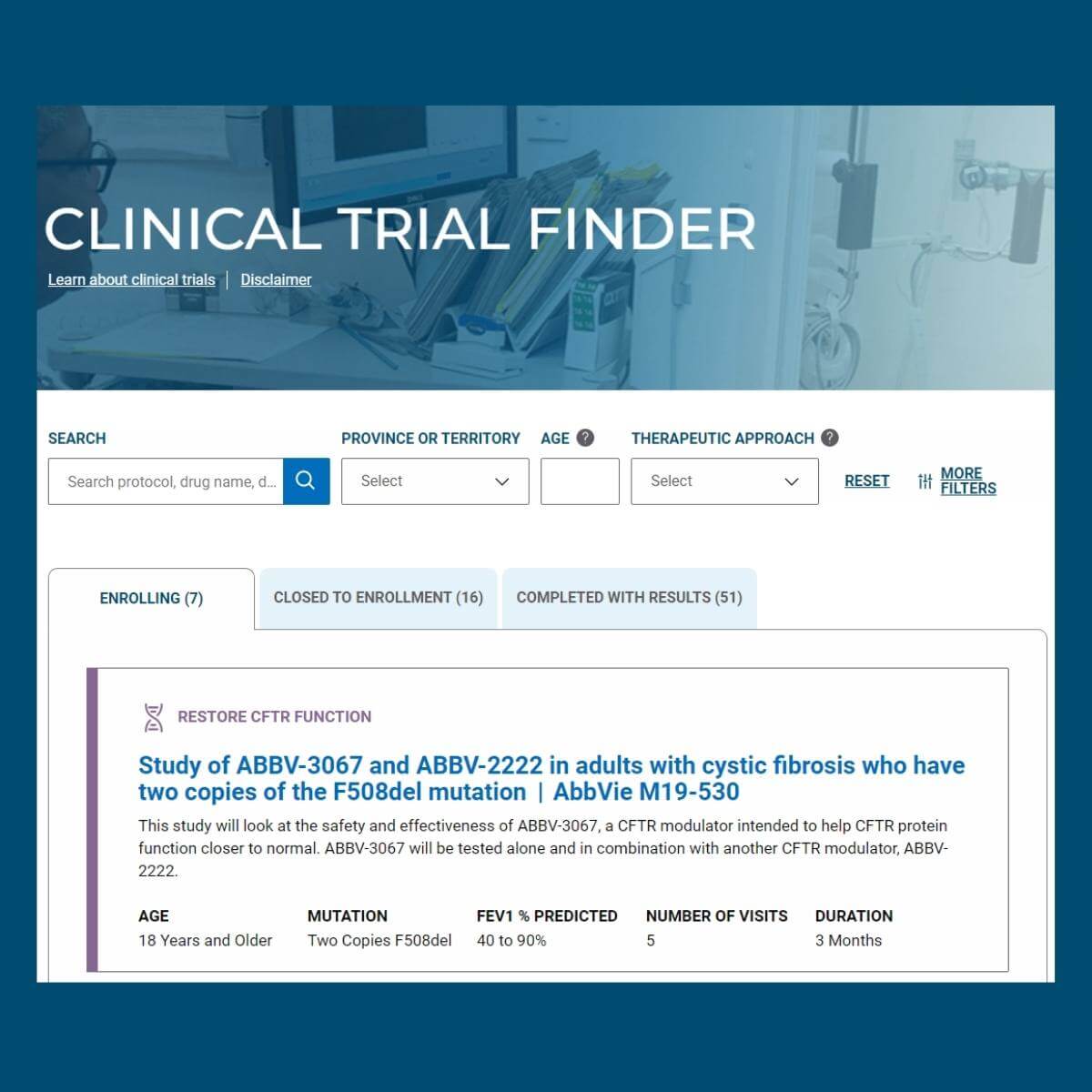
Our revamped Clinical Trial Finder tool makes it easy for you to search for clinical trials that are currently enrolling across Canada, find information on previous trials and their results! We worked with our partners at the Cystic Fibrosis Foundation and their Therapeutics Development Network (TDN) to bring this tool to our community – we hope that it helps you to find trials you are eligible for and interested in!
Currently, the clinical trial finder is available in English, the French tool will be coming in the future.
 |
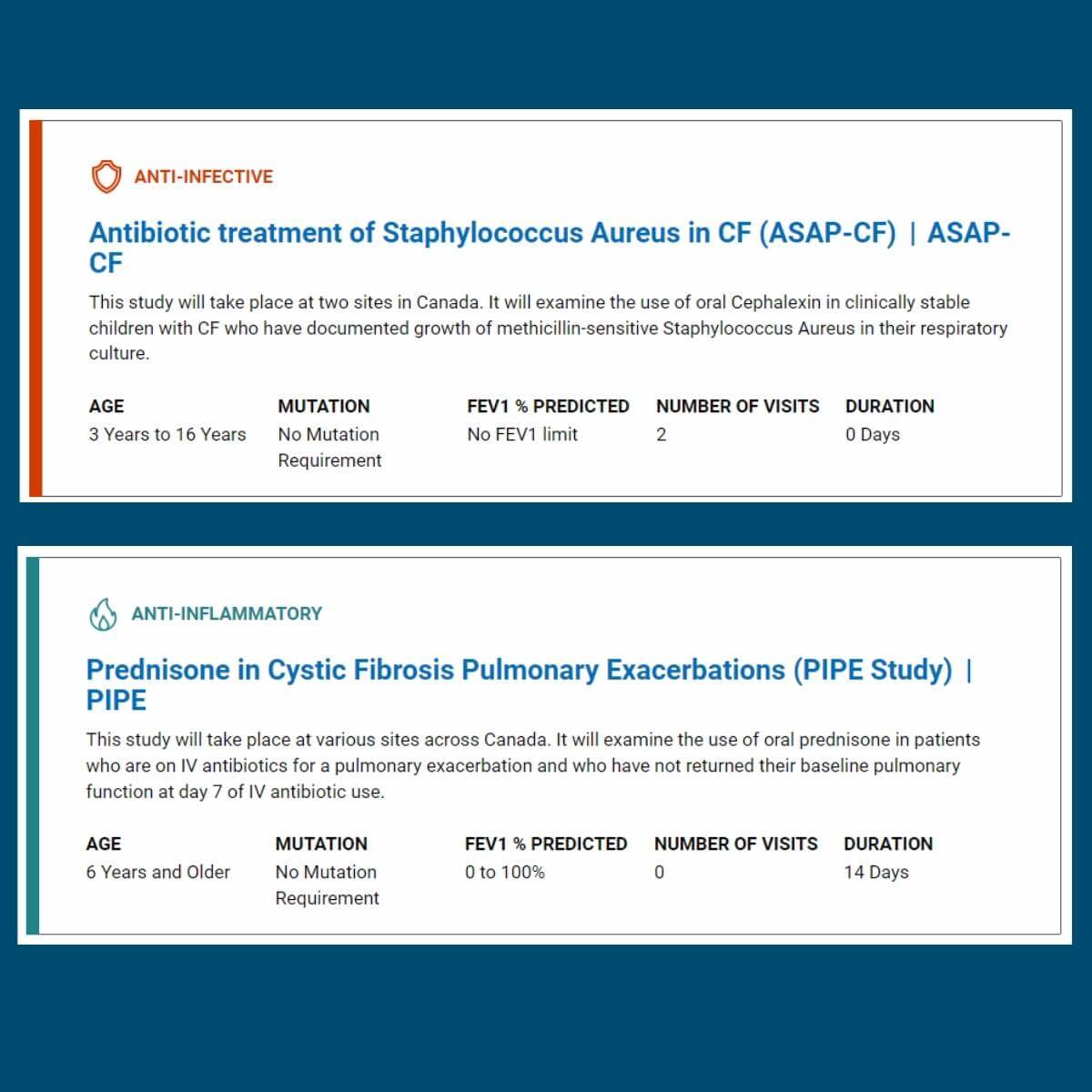 |
Clinical trials test potential new drugs, treatments and devices to assess how well they work, and whether they offer benefits over currently used drugs, treatments or therapies. Drugs and devices are first tested in research laboratories where experiments are done on animals and human cells. If the research is successful and the drug or treatment is deemed safe, it will go into the first phase of a clinical trial. View the Clinical Trial Road Map to see what happens throughout the different phases of a clinical trial. Looking for information on genetic and/or sweat testing? Find information here.

CYSTIC FIBROSIS CANADA ACCELERATING CLINICAL TRIALS NETWORK (CF CanACT)
In keeping with its vision of a world without cystic fibrosis, Cystic Fibrosis Canada has established a Canadian clinical trials network, Cystic Fibrosis Canada Accelerating Clinical Trials (CF CanACT). World-class clinical trials are an integral part of the process that brings new therapeutics and better care to Canadians who are living with cystic fibrosis. The establishment of CF CanACT will help to facilitate the development of these new treatments for cystic fibrosis, as well as increase capacity and enhance participation of people with cystic fibrosis in clinical trials.
The CF CanACT network presently consists of ten sites across Canada (two in Montréal, two in Toronto, two in Vancouver, one in Calgary, one in Saskatoon, one in Quebec City, and one in Halifax.) In 2020, the network officially expanded to include four additional four sites: one each in Quebec City, Saskatoon, Halifax and Montreal which brought us up from six sites, to ten. Anyone with cystic fibrosis living anywhere in Canada is eligible to be referred to one of these sites to participate in a clinical trial, and can participate if they meet the selection criteria of the specific trial.
The investigators and research coordinators from the ten sites, along with an adult patient representative and a CF parent, collaborate to review research protocols, standardize outcome measures between sites, and ensure that clinical trials are feasible to perform and relevant to patients. Do you have a question about clinical trials or CF CanACT? Please send an email to clinicaltrials@cysticfibrosis.ca.
Annual Report
Want to learn more about clinical trials? Check out Cystic Fibrosis Canada’s Clinical Trial information Kit for everything you need to know to make an informed decision about whether you CanACT and participate in clinical trials in Canada.
DOWNLOAD INFORMATION KIT Subscribe to CF Canada’s Clinical Trial Mailing ListClinical trial resources
TESTIMONIALS
Enrolling in a study takes a degree of courage. You’re forced to make some tough decisions and you’re jumping into the unknown which can be scary, however, I would participate in another clinical trial in a heartbeat. Whether the outcome is positive or not, trials have the power to impact our future and that’s more than worth it to me.
A tough part of living with CF is the isolation, but by participating in clinical trials, I’ve been given opportunities to create a network of people who understand CF, look out for me, and provide me with information. I’ve been able to grapple with CF better as a result.
"I have been able to participate in one clinical trial, and I feel that it is important to participate in these trials whenever possible. There is a limited population of people living with CF, so if there is something that I can do to help future generations to live better and longer, I think it's worth doing that."
I think it’s important to participate in clinical trials because it can help other people to stay healthy and I like that I am helping other people and myself.
"Enrolling in clinical trials is an odd mixture of stress and excitement. Leading into the trials you're just hoping that in the last month nothing goes wrong and that you can stay stable. Then once you are finally randomized, it's cautious optimism. Not knowing whether you're on the drug or not, you pay attention to every little symptom and sign of improvement while trying not to get your hopes up. Some clinical trials are more time consuming than others, especially when they require the sleep index and CFQR daily. But overall it’s a positive experience, especially if you manage to get a boost in health out of the deal."
Between managing parenting, work, after-school programs, CF treatments, and life, adding more to our schedule was daunting at first. I was really surprised to learn that participating in a clinical trial wasn’t as big of a time commitment as I thought it would be. Sometimes we were even able to coordinate our participation with clinic visits, which made it really convenient to participate.
To find more patients testimonials, check out our Youtube channel here.

